As the use of electric and hybrid vehicles increases globally, the need for safe and high-performing battery technologies becomes paramount. Engineers are focused on developing batteries that offer increased safety, energy capacity, scalability, and reduced degradation over time. One promising avenue of research is rechargeable multivalent metal batteries that employ multivalent ions such as magnesium (Mg) and calcium (Ca) as anode materials. These batteries have the potential for high energy densities if the right combination of anodes, cathodes, and electrolytes is developed.
Challenges in Electrolyte Development
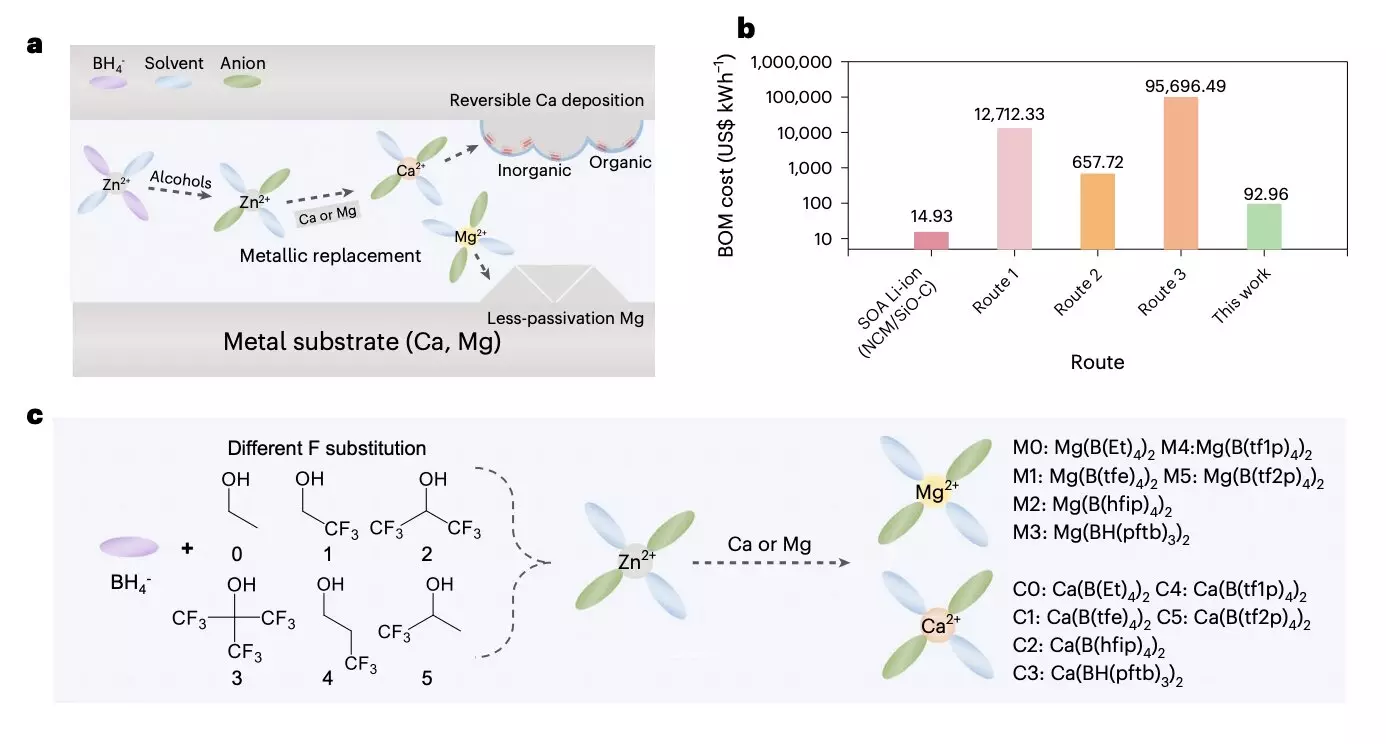
Researchers have identified cost-effective anode materials for multivalent metal batteries in recent years. However, finding suitable electrolytes poses a significant challenge. Many proposed electrolytes are either difficult to source or require complex synthesis processes, making large-scale fabrication impractical. To address these limitations, a team of researchers from Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology introduced a groundbreaking method for highly performing and scalable electrolytes for multivalent metal batteries.
The research team’s method involves several steps. Initially, a chemical reaction was initiated between an affordable and easily attainable Zn(BH4)2 precursor and different fluoroalcohols, resulting in target anions with various branched chains. These anion solvates then reacted with low-cost metal foils to produce target solvation structures. In order to maintain stable battery cycling and suppress solvent decomposition, the researchers proposed the formation of a passivation layer using two types of Ca solvates.
By adjusting the precursor chain length and F-substitution degree, the researchers were able to fine-tune anion participation in the primary solvation shell. This optimization led to a completely dissociated Mg organoborate electrolyte with enhanced electrochemical kinetics and high current endurance. Additionally, the Ca organoborate electrolyte with strong coordination and B-H inclusion offered a stable solid-electrolyte interphase with high coulombic efficiency.
The researchers utilized their method to create a high-loading battery prototype based on Mg/S, achieving a remarkable energy density of 53.4 Wh/kg. The prototype consisted of a 30 μm Mg anode, a low electrolyte/sulfur ratio, and a modified separator/interlayer. Initial testing demonstrated promising results, highlighting the potential of this approach to develop cost-effective electrolytes for multivalent metal batteries.
This groundbreaking method opens doors for the creation of various reversible electrolyte systems that rely on more affordable materials and simpler processing strategies. These new electrolytes have the potential to create scalable and safe multivalent metal batteries with higher energy densities. The advancements made by the researchers at Zhejiang University, the ZJU-Hangzhou Global Scientific and Technological Innovation Center, and Dalian University of Technology offer exciting possibilities for the future of battery technology.
The development of safe and high-performing battery technologies is essential as the use of electric and hybrid vehicles continues to rise. One area of focus is the improvement of multivalent metal batteries, which rely on innovative electrolytes. The method introduced by researchers has the potential to revolutionize the way electrolytes are developed, leading to more affordable and scalable energy storage solutions. Further research is needed to refine and optimize this approach, but the future of multivalent metal batteries looks promising.

Leave a Reply