Lithium-metal batteries have long been hailed as the next frontier in battery technology due to their significantly higher energy densities compared to lithium-ion batteries. However, issues such as short lifespan and the growth of lithium dendrites have hindered their widespread adoption in the market. Recent research conducted by Prof. Shuhong Jiao and her team at the University of Science and Technology of China has introduced a new electrolyte design that could potentially revolutionize the performance of lithium-metal batteries.
One of the major obstacles facing lithium-metal batteries is their short cycle life, typically limited to around 50 cycles, in stark contrast to the approximately 1,000 cycles that commercial lithium-ion batteries are capable of. The growth of lithium dendrites, as well as the high reactivity of lithium-metal and high-voltage transition metal cathodes, contribute to the constant degradation of the electrolyte. This degradation occurs due to the instability of the interfaces between the electrolyte and electrodes, preventing the stabilization seen in lithium-ion batteries. As Prof. Jiao notes, the performance of lithium-metal batteries is still far from satisfactory, with efforts to achieve higher energy densities and longer lifespans ongoing.
Prof. Jiao and her colleagues developed an electrolyte five years ago to stabilize the interfaces between the anode-electrolyte and cathode-electrolyte in lithium-metal battery cells, effectively suppressing the degradation of the electrolyte. Their design was informed by a deep understanding of the physicochemical processes within lithium-metal batteries and aimed to utilize inexpensive components for practical applications. Building on previous research in the field, the team collaborated to create a new class of electrolytes that address the challenges faced by lithium-metal batteries.
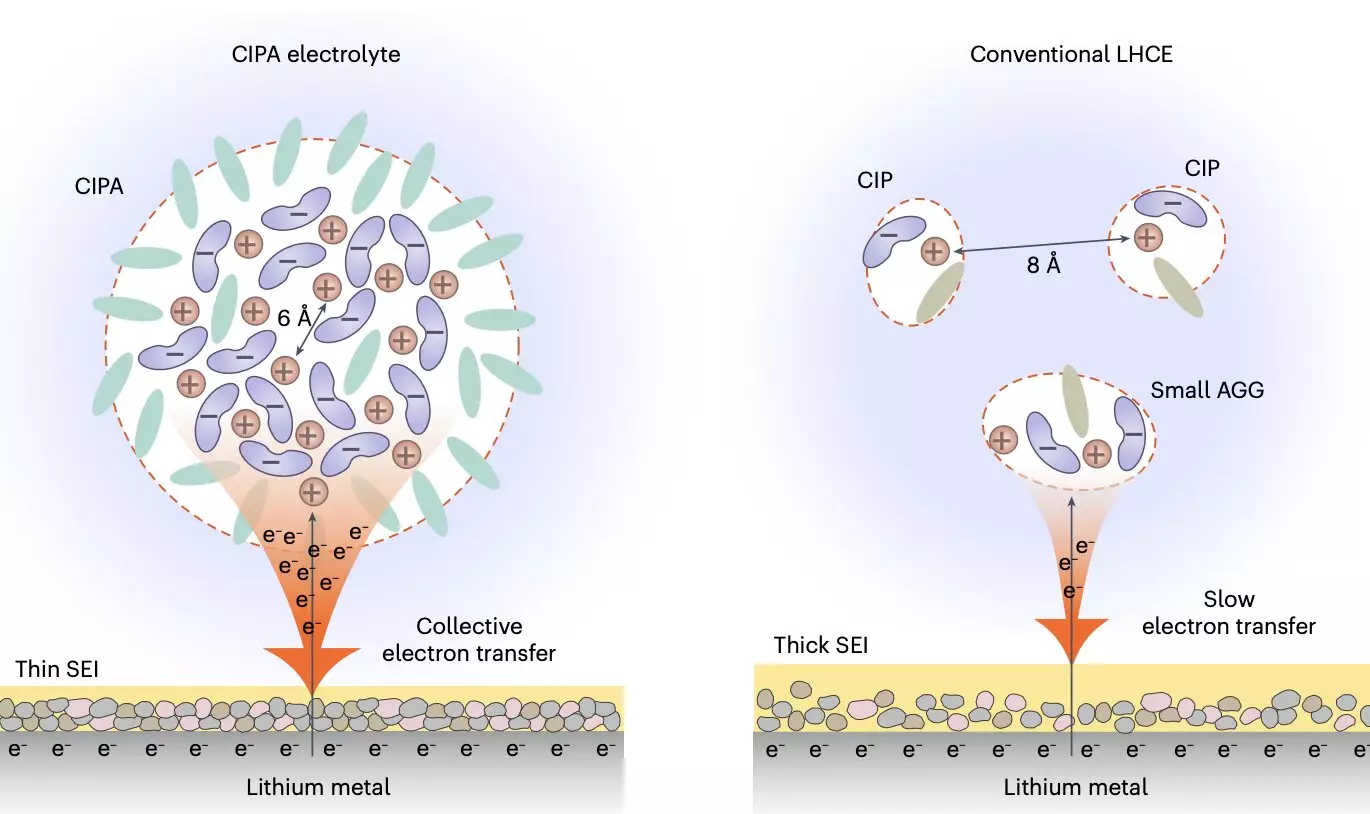
The recent study by Prof. Jiao’s team focused on the tuning of the electrolyte’s solvation structure at the mesoscopic level, particularly on the interaction between ion pairs that form the electrolyte’s aggregate structure. Their electrolyte design, known as the compact ion-pair aggregate (CIPA), features densely packed lithium-anion ion pairs with coordination bonding between each other. This structure promotes the rapid reduction of anions on the lithium-metal anode surface, forming a stable solid-electrolyte interface (SEI) that inhibits further electrolyte decomposition and dendrite growth. Additionally, the electrolyte exhibits good oxidative stability and suppresses the dissolution of transition metal elements from the cathode, ensuring stable cycling over an extended period.
The promising results obtained with the new electrolyte design indicate a significant advancement in the field of lithium-metal batteries. The 500 Wh/kg lithium-metal pouch cell created using this electrolyte retained 91% of its energy after 130 cycles, showcasing its potential for practical applications. Prof. Jiao and her team aim to further extend the cycle life of the cell to over 1,000 cycles while exploring new battery systems with even higher energy densities. This breakthrough in electrolyte design opens up new possibilities for the future of lithium-metal batteries, offering a path towards higher performance and longevity in energy storage technologies.

Leave a Reply