In a remarkable advancement in the field of physics, researchers at TU Wien have successfully generated laser-synchronized ion pulses that last for less than 500 picoseconds. This pioneering work, recently published in *Physical Review Research*, opens the door to new methods of observing and analyzing chemical processes on material surfaces. The implications of this research stretch beyond mere academic interest; it holds the potential to revolutionize various industrial applications, scientific research, and our fundamental understanding of chemical interactions.
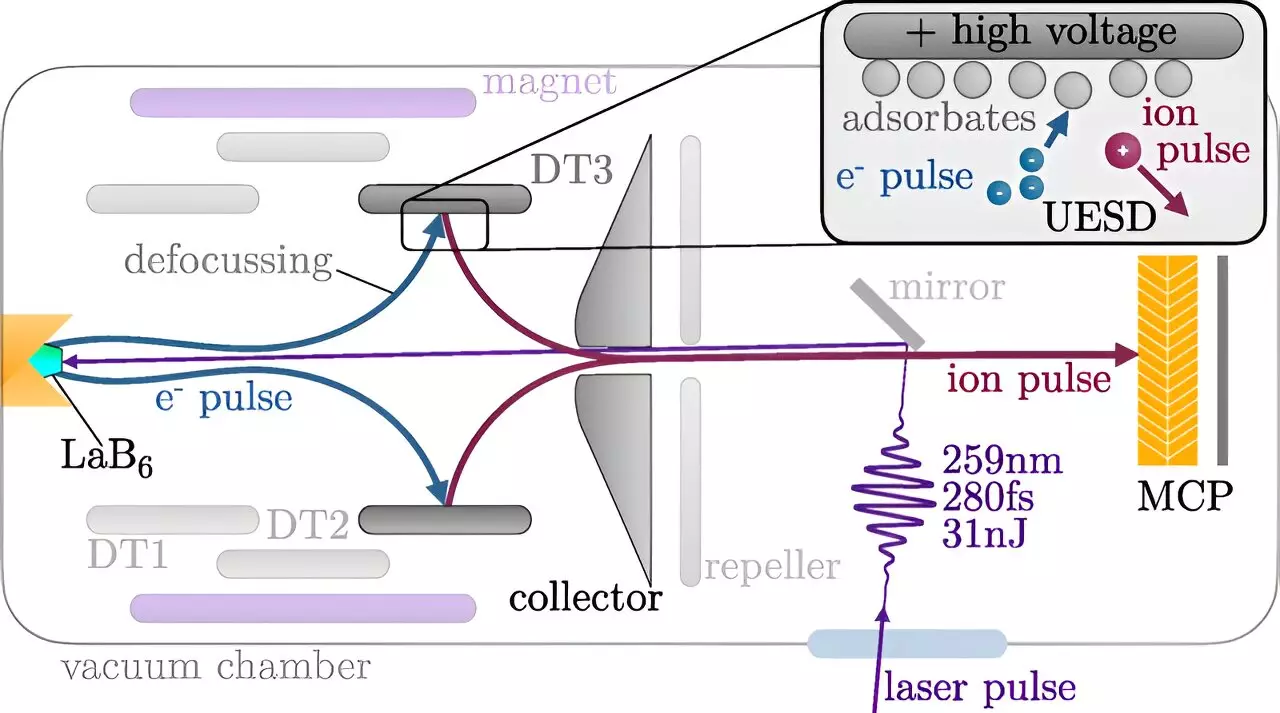
The principle behind this technological leap hinges on the necessity for extremely short exposure times in capturing fast events, akin to the usage of rapid shutter speeds in photography. In physics, laser pulses are predominantly utilized to visualize atomic processes, but ion pulses now have an opportunity to take center stage. According to Prof. Richard Wilhelm from TU Wien’s Institute of Applied Physics, the new technique allows for the generation of intense, ultra-brief pulses of charged particles, enabling controlled impacts on target surfaces. This advancement facilitates the real-time observation of chemical processes, providing insights that were previously unattainable.
Historically, the use of ion beams has involved analyzing the resultant changes after a reaction has taken place. Prof. Wilhelm articulately explains that traditional methods provide a snapshot of the final product rather than the dynamic processes leading to it. The crux of the challenge lay in producing ion pulses so brief that they could follow the actual timeline of reactive interactions.
The newly achieved ion pulses are significant due to their duration of less than 500 picoseconds—an interval that, while exceedingly brief on a human scale, opens new opportunities for surface analysis. For perspective, this duration allows light to travel a mere 15 centimeters, yet it’s considerably longer than the attosecond pulses that currently represent the shortest laser outputs. Nevertheless, the 500 picosecond timescale is ideal for studying surface behaviors, striking a balance that enables effective observation of transient chemical reactions.
Achieving this feat required a multi-stage process whereby an initial laser pulse strikes a cathode, leading to electron emission. These electrons are then accelerated towards a stainless steel target. Wilhelm describes how certain atoms, especially hydrogen and oxygen, preferentially adhere to this stainless steel surface. Upon impacting the surface, electrons free some of these attached atoms, resulting in a fraction that becomes ionized while others remain neutral.
Following this initial ion generation, electric fields can be employed to distinguish between the ionized and neutral atoms, directing a precisely controlled short ion pulse for analysis. This meticulous timing is crucial—by initiating the ion generation with a laser pulse, researchers can exactly synchronize when the ion pulse strikes the surface under investigation, thereby capturing real-time data during ongoing chemical reactions.
The groundbreaking technique is versatile; although it currently utilizes protons—the simplest ions—it also has the potential to generate other types of ionized particles, such as carbon or oxygen ions. Wilhelm notes that the selection of ions boils down to the attachment of specific atoms to the stainless steel layer; this fine control empowers researchers to tailor the experimental setup for various analyses.
Looking ahead, the team at TU Wien is not resting on their laurels. Plans are already underway to further minimize the duration of ion pulses, employing specially designed alternating electromagnetic fields. This advancement could yield even more powerful observation capabilities, pushing the envelope of what is currently feasible in ultrafast science.
Moreover, the integration of this ion pulse technology with ultrafast electron microscopy holds promise for deeper insights into surface chemistry. By combining these methodologies, researchers can explore an even broader spectrum of physical and chemical phenomena, facilitating a more comprehensive understanding of material properties and reactions.
The development of laser-synchronized ion pulses under 500 picoseconds marks a significant milestone in the study of chemical processes at material surfaces. TU Wien’s innovative efforts exemplify how combining traditional methods with cutting-edge technology can lead to revolutionary outcomes in scientific research. As these techniques mature, we can anticipate a wealth of new discoveries that could reshape our approach to material science, chemistry, and beyond, ultimately pushing the boundaries of what we understand about the microscopic world.

Leave a Reply