Photosynthesis is one of nature’s remarkable processes, allowing plants, algae, and certain bacteria to convert sunlight into chemical energy. This biological phenomenon mirrors the technology behind solar panels, where photovoltaics convert light into electricity. At the heart of both procedures lies a fundamental principle: the movement of electrons. This intricate dance of charge transfer occurs at the molecular level and is crucial for both the sustenance of life and the advancement of renewable energy technologies. Understanding these processes better not only sheds light on biological systems but also propels innovation in energy generation.
The Quest for Ultrafast Understanding
The realm of molecular electron dynamics is not just a playground for theoretical physicists and chemists; it is a vibrant field with practical implications that could revolutionize technology. When a molecule absorbs light, it experiences a rapid redistribution of electron density—an ultrafast process that eludes traditional measurement techniques. Harnessing advanced methods, we can now explore these instantaneous events with time resolution that spans femtoseconds (10^-15 seconds) to attoseconds (10^-18 seconds). Despite significant technological advancements, a comprehensive understanding of electron and charge transfer after photoionization remains elusive. This gap in knowledge drives ongoing research.
New Insights from Cutting-Edge Research
A groundbreaking study published in *Nature Chemistry* by researchers from Politecnico di Milano and various Madrid institutions expands our understanding of these ultrafast processes. By employing attosecond extreme-ultraviolet (XUV) pulses, the team scrutinized molecular systems to gain insights into the intricate interactions between electrons and their atomic nuclei. Their focus on nitroaniline molecules revealed transformative dynamics occurring at timescales previously considered unattainable, advancing our grasp of fundamental chemical processes.
Investigating Charge Transfer with Precision
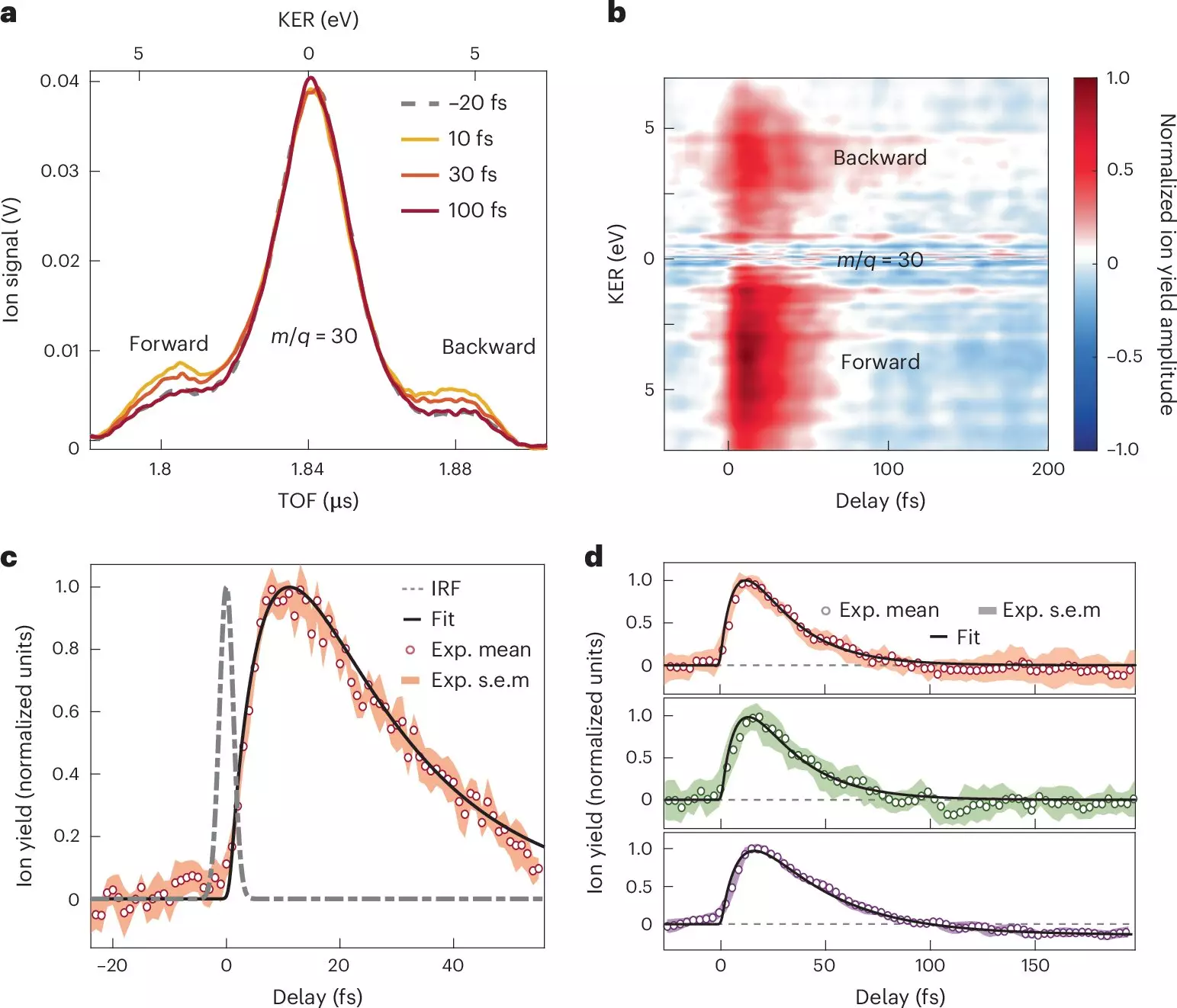
Utilizing a sophisticated combination of attosecond XUV-pump and femtosecond infrared-probe spectroscopy, the researchers generated a coherent experimental setup to observe charge transfer dynamics. They meticulously documented the stages of electron migration when nitroaniline was subjected to XUV pulses. Remarkably, they found that electron transfer from the amino group to the molecular cation transpires in under 10 femtoseconds. This intricate transfer is not just a straightforward handoff; it involves synchronized movements of both electrons and nuclei, suggesting a tightly coupled dynamic system.
In addition to the initial electron transfer, the findings unveiled a subsequent relaxation phase that develops over less than 30 femtoseconds. During this phase, the nuclear wave packet disperses within the excited electronic states of the newly formed cation. These observations elucidate the crucial role of electron-nuclear coupling, offering new insights into the mechanisms of donor-acceptor interactions in organic molecules. The implications of these findings extend far beyond academic exploration; they have the potential to reshape our understanding of organic electronic materials and improve device efficiencies.
This cutting-edge research not only exposes fundamental principles governing charge transfer but also aligns with longstanding theoretical frameworks regarding molecular dynamics. The outcomes challenge and refine classical models often used to predict charge migration. By revealing the temporal landscape of charge transfer events, this work opens new avenues for both theoretical development and experimental inquiry, paving the way for innovations in attosecond science and its applications.
In an era where energy efficiency and molecular engineering are at the forefront of scientific exploration, understanding the subtleties of electron and charge transfer processes has never been more critical. The innovative methodologies illustrated in this research mark a significant leap forward in ultrafast science. As we delve deeper into these molecular dynamics, the prospect of applying such knowledge holds promise not only for improving energy technologies but also for harnessing and manipulating chemical processes on unprecedented timescales. The quantum realm merely begins to reveal its mysteries, inviting further exploration and discovery.

Leave a Reply