The pursuit of sustainable energy solutions is increasingly pressing in the context of climate change and fossil fuel depletion. As the global community seeks alternatives to traditional hydrocarbons, one innovative approach is the utilization of carbon dioxide (CO2) to produce valuable fuels. Researchers at the University of Michigan have made groundbreaking strides in this domain with a novel artificial photosynthesis system capable of efficiently converting CO2 into hydrocarbons. This article delves into the technology, its potential applications, and the broader implications for sustainable fuel production.
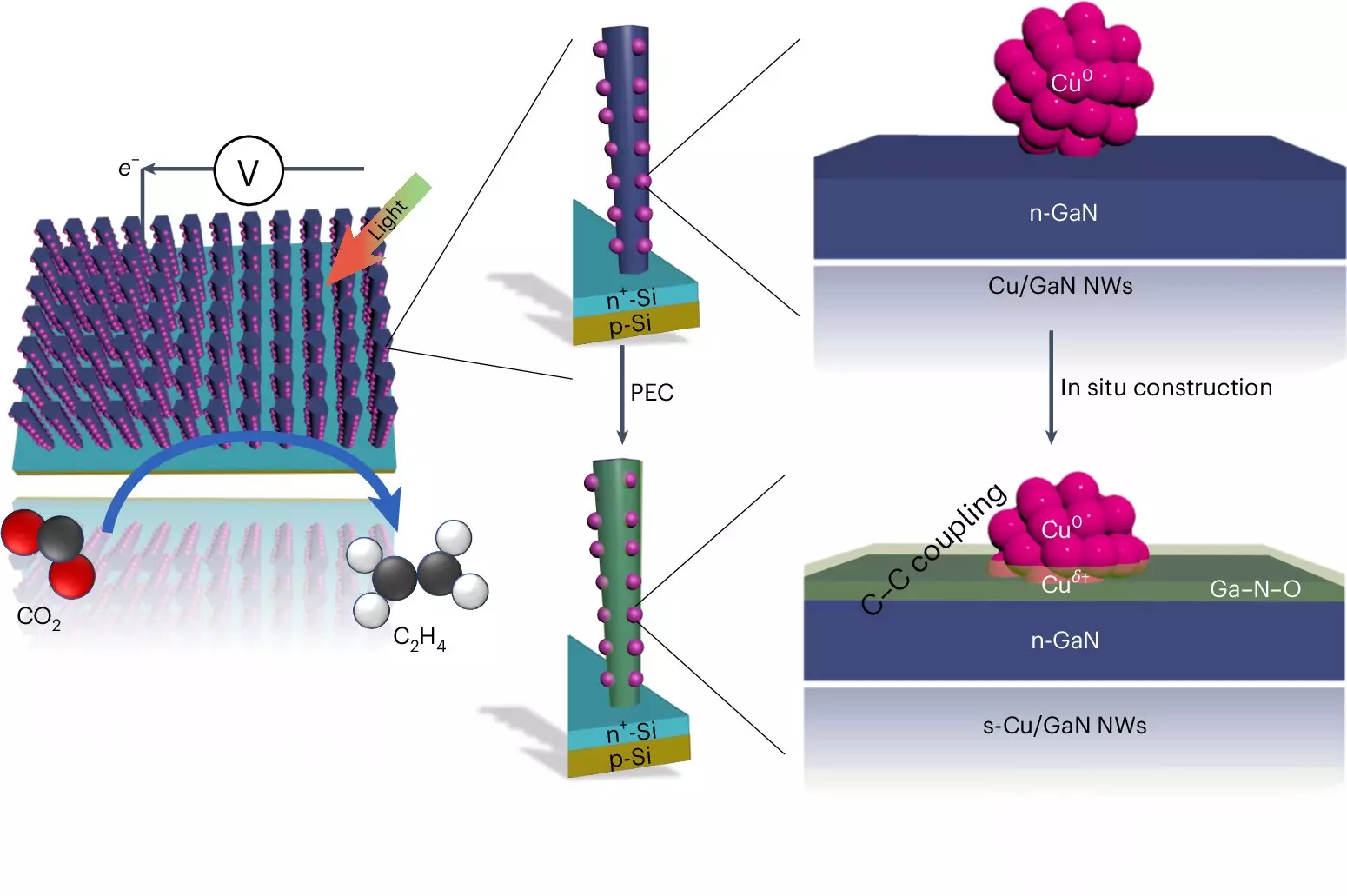
At the heart of the University of Michigan’s development is the ability to bind carbon atoms together, enabling the conversion of CO2—often considered a waste product of industrial processes—into essential hydrocarbons. Their artificial photosynthesis system stands out due to its remarkable efficiency and longevity in generating ethylene, a compound ubiquitously used in the production of plastics. Professor Zetian Mi, a key figure in this research, highlights that the performance of this system surpasses conventional methods by a factor of five to six, setting a new benchmark in the field.
The current production of ethylene primarily relies on fossil fuels and is characterized by energy-intensive processes that contribute significantly to greenhouse gas emissions. By transforming CO2 and water into ethylene, the Michigan team’s approach not only harnesses otherwise harmful emissions but also provides a sustainable pathway for plastic production. This innovative technology could redefine industries traditionally reliant on oil and gas, ushering in a new era of eco-friendly manufacturing practices.
The transformative nature of this system lies in its advanced design. The core mechanism utilizes gallium nitride (GaN) nanowires, which are minuscule in size—just 50 nanometers in width—complemented by a silicon substrate. This unique configuration enables effective capture and conversion of light energy, which is vital for the chemical reactions that follow. When exposed to sunlight, the nanowires facilitate the splitting of water molecules, generating hydrogen and oxygen.
Simultaneously, copper clusters integrated into the system play an essential role in the chemical transformation of CO2 into ethylene. The creation of hydrogen, together with carbon monoxide from the captured CO2, initiates a process where these molecules combine into ethylene. The efficiency of this reaction is enhanced significantly when the team optimized the interactions at the essential catalysts’ interface.
The ingenuity of this design enhances the overall energy conversion process with approximately 61% of the electrons generated contributing to ethylene production—a striking contrast to previous systems that struggled with lower efficiencies and limited operational longevity.
The ability for this device to maintain its functionality is another critical achievement. Unlike alternative catalysis approaches, which often degrade rapidly, the University of Michigan’s system operated continuously for 116 hours without reduction in performance, with extended trials reaching up to 3,000 hours. This resilience can be attributed to the synergistic relationship between the gallium nitride and oxygen presence during the water-splitting phase, allowing the system to undergo a self-healing process.
Bingxing Zhang, a co-author of the study, notes the aspirations of further enhancing the technology to yield more complex hydrocarbons, such as propanol. This trajectory could significantly expand the range of sustainable transport fuels, moving beyond commoditized products like ethylene toward more versatile liquid fuels.
The implications of this research are profound, extending beyond merely converting CO2 into hydrocarbons. As society grapples with the need for more sustainable practices, technologies like artificial photosynthesis could redefine energy production matrices worldwide. The transition toward a circular economy—where carbon is recycled and reused rather than emitted—marks a pivotal shift in addressing the climate crisis.
Moreover, the potential for producing liquid fuels from CO2 could provide a lifeline for existing transportation infrastructures designed around liquid fuel usage, allowing for more gradual, manageable transitions to sustainable energy sources.
The University of Michigan’s advancements in artificial photosynthesis reflect significant progress toward sustainable fuel production. By reimagining CO2 as a feedstock, researchers are paving the way for innovative approaches that promise to mitigate environmental impacts while fulfilling the growing energy demands of a modern society. The journey is just beginning, but the path forward holds immense promise for transforming how we think about carbon emissions and energy use globally.

Leave a Reply