Bipolar membranes, commonly used in various energy technologies such as electrolyzers and hydrogen fuel cells, have been a subject of interest for many researchers and companies. However, the underlying working principles of these membranes and the ion solvation kinetics involved have remained a mystery for a long time. The lack of a comprehensive understanding has hindered the full potential of these membranes in different devices. Recently, researchers at the Fritz Haber Institute of the Max Planck Society shed light on these fundamental principles in a study published in Nature Energy.
The study conducted by the research team led by Sebastian Oener and Carlos Gomez Rodellar involved overcoming several experimental challenges. Setting up a system to study the kinetics of bipolar membranes while preventing interference from electrolyte ions was a crucial aspect of their work. This required the design of a system that could apply physical pressure on the membrane electrode assembly and allow for the manipulation of temperature and humidified gases for analysis. The team also focused on extracting bias-dependent activation entropy and enthalpy to gain a better insight into the functioning of these membranes.
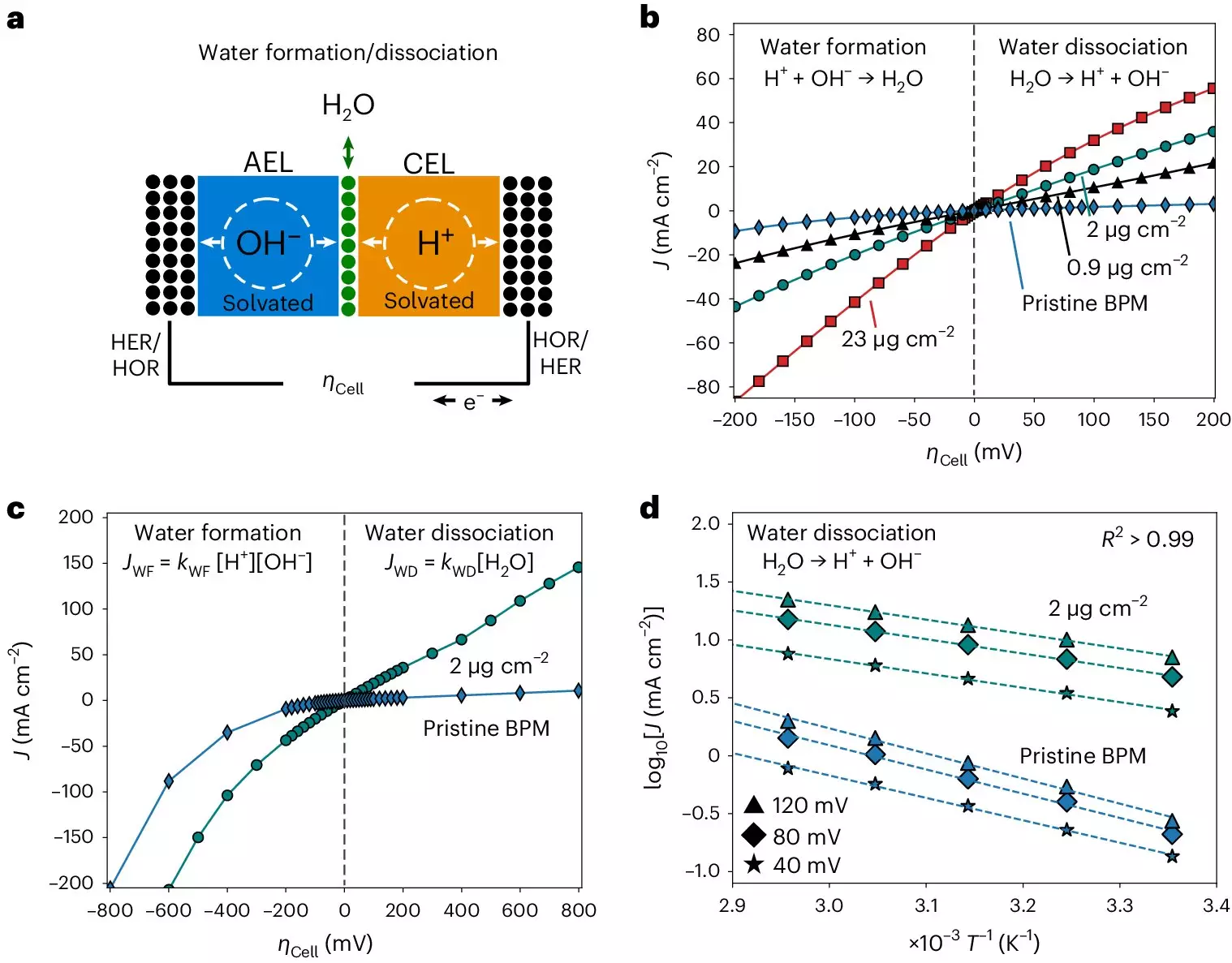
Through their measurements and analysis, the researchers uncovered bias-dependent relationships between activation entropy and enthalpy in the bipolar junction of the membranes. They also observed solvation kinetics characteristics that were not influenced by the catalysts’ chemical composition but rather by entropic changes in the interfacial electrolyte. These findings have significant implications for the future development of bipolar membranes in applications such as electrodialysis, CO2 electrolyzers, and hydrogen fuel cells. The potential for improved performance in these technologies is promising based on the insights gained from this study.
Beyond the realm of bipolar membranes, the research conducted at the Interface Science Department of the Fritz Haber Institute has broader implications for electrolysis and electrocatalysis. The entropic changes identified in the study can guide the design of new electrocatalysts to initiate specific chemical reactions essential for green hydrogen production from alkaline electrolytes. The connection between water dissociation and ion solvation at electrocatalyst interfaces highlights the interconnectedness of various energy conversion processes and the importance of understanding fundamental physics at play.
While the recent study has provided valuable insights into the working principles of bipolar membranes, there are still open questions and areas for further exploration. The researchers aim to address fundamental questions such as the water formation reaction in forward-running bipolar membranes and explore various applications in collaboration with other research groups. Projects involving different fuel cell technologies and electrodialysis systems are underway, emphasizing the diverse potential applications of bipolar membranes in the energy sector. Additionally, ongoing research in ion solvation in electrocatalysis is expected to yield more significant discoveries in the near future.
The recent discoveries in bipolar membranes research have paved the way for a deeper understanding of these critical components in energy technologies. The implications extend beyond the membranes themselves, offering insights into broader fields such as electrolysis and electrocatalysis. By unraveling the mysteries of bipolar membranes, researchers are opening new avenues for innovation and the development of sustainable energy solutions.

Leave a Reply