Fuel cells have emerged as a promising energy-conversion solution that generates electricity through electrochemical reactions without the harmful effects of combustion. This technology has the potential to power a wide range of applications, from electric vehicles to industrial machines, while reducing pollution levels and carbon emissions. However, the main limitation of current fuel cell designs lies in their reliance on expensive materials and precious metal catalysts, which hinder their widespread adoption.
Anion-exchange-membrane fuel cells (AEMFCs) have been proposed as a solution to address the cost barriers associated with traditional fuel cell technologies. These cells are based on Earth-abundant and low-cost catalysts, making them more affordable and accessible. Despite their potential advantages, most non-precious metals used in AEMFCs are prone to self-oxidation, leading to irreversible failure of the cells over time. This inherent problem has limited the efficiency and longevity of AEMFCs, hindering their commercial viability.
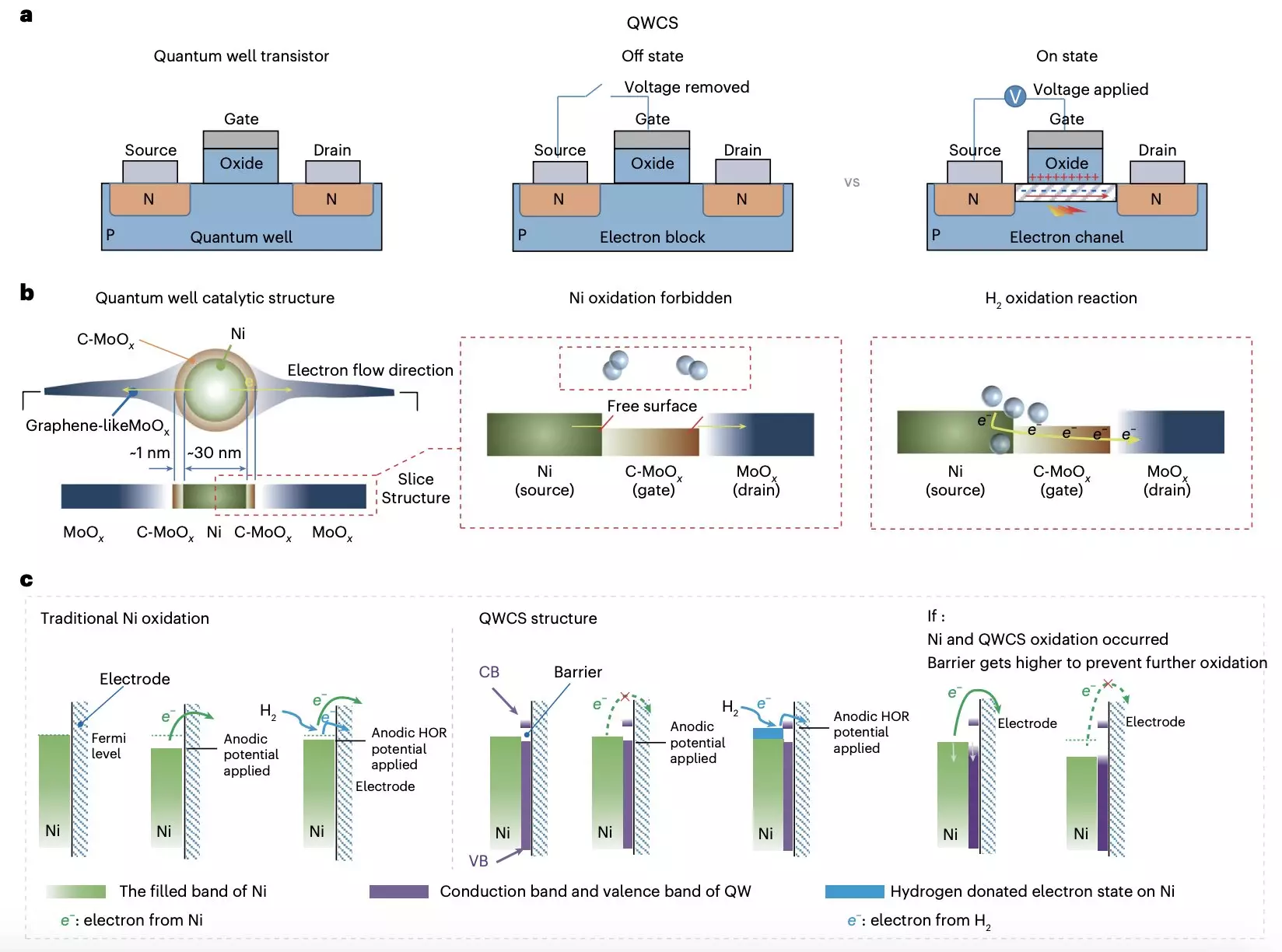
Researchers at Chongqing University and Loughborough University have recently introduced a revolutionary strategy to prevent the oxidation of metallic nickel electrocatalysts in AEMFCs. This innovative approach, detailed in a paper published in Nature Energy, incorporates a newly designed catalytic structure known as a quantum well-like catalytic structure (QWCS). This structure consists of quantum-confined metallic nickel nanoparticles that effectively prevent self-oxidation and enhance catalytic activity.
The QWCS created by the researchers features a unique composition, with Ni nanoparticles confined within a heterojunction made up of carbon-doped MoOx (C-MoOx) as the low energy valley and amorphous MoOx as the high energy barrier. This design, known as Ni@C-MoOx, enables selective transfer of external electrons produced during the hydrogen oxidation reaction, ensuring the stability and longevity of the catalyst. By preventing the transfer of electrons from the Ni catalyst into the QWCS valley, the catalyst remains robust against electro-oxidation, thereby protecting the fuel cells from degradation and failure.
The Ni@C-MoOx catalyst demonstrated exceptional stability and efficiency, maintaining high catalytic activity even after 100 hours of continuous operation under harsh conditions. The anode-catalyzed alkaline fuel cell created using this catalyst achieved remarkable results, with a specific power density of 486 mW mgNi-1 and no decline in performance following repeated shutdown-start cycles. This breakthrough in catalytic structure design showcases the potential of QWCS to revolutionize the development of cost-effective AEMFCs that are more reliable and long-lasting.
The introduction of quantum well-like catalytic structures represents a significant advancement in the field of fuel cell technology. By leveraging quantum confinement to prevent the electro-oxidation of non-precious metals, researchers have unlocked a new paradigm in catalyst design that could pave the way for more sustainable and efficient energy solutions. The breakthrough achieved by the team of scientists at Chongqing University and Loughborough University highlights the importance of innovation and collaboration in driving progress towards a cleaner and greener future.

Leave a Reply