In recent years, the intersection of physics and biology has garnered significant attention, particularly in the field of condensed matter physics. Researchers at São Paulo State University (UNESP) in Brazil have made strides in understanding protein compartmentalization within cells using theoretical frameworks typically reserved for physical systems. Their study delves into the concept of phase separation — a phenomenon commonly seen in materials science — suggesting that similar processes govern biological dynamics at the cellular level.
The research, led by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, draws parallels between the behavior of particles in condensed matter systems and the ways proteins organize within biological cells. This innovation has the potential to unlock new avenues for understanding cellular processes, thereby enhancing our grasp of complex biological phenomena, including the origins of life itself.
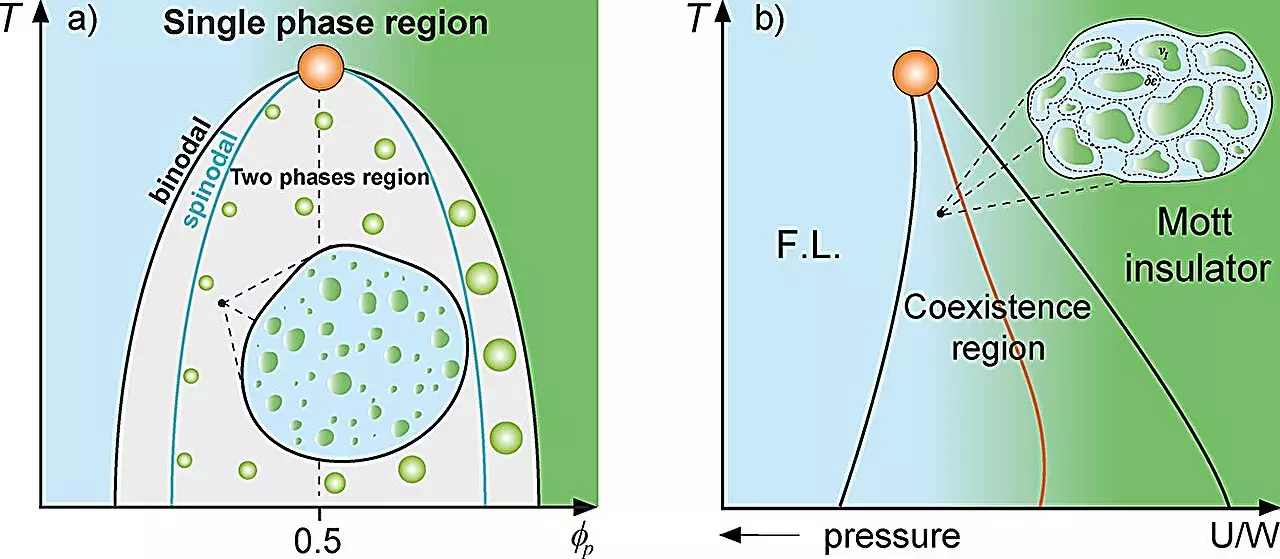
Utilizing the established concepts of Griffiths phases, which typically describe emergent properties in magnets, the study presents a ‘Griffiths-like phase’ in cellular biology. In a similar manner that magnetized and non-magnetized regions influence the dynamics in ferromagnetic and paramagnetic systems, the researchers propose that protein droplets inside cells create distinct microenvironments that influence cellular function and dynamics.
These protein droplets arise through a process known as liquid-liquid phase separation, wherein proteins condense into liquid-like droplets within the cellular milieu. This spatial compartmentalization allows for localized concentration of biomolecules and can significantly affect biochemical reactions. The researchers effectively use thermodynamic principles, including the Grüneisen parameter and Flory-Huggins theory, to analyze how these factors interplay in a cellular context.
One of the most exhilarating implications of the study is its potential link to the origin of life, connecting the behavior of coacervates — droplets formed by organic molecules in aqueous solutions — to the very roots of biological evolution. In the 1930s, biologist Aleksandr Oparin postulated that these primitive structures provided an environment conducive for early biological processes, thereby serving as a precursor to life. By framing protein droplets as analogous to Oparin’s coacervates, the researchers offer a compelling theory that the slow dynamics characterizing these regions allowed for stability and evolutionary potential.
Interestingly, the study also touches upon the concept of homochirality — the predominance of one chiral form of a molecule over another — which is pivotal in biochemical processes. The stabilization mechanisms provided by liquid-liquid phase separation may have played a crucial role in fostering environments where homochiral molecules could thrive, thereby influencing biochemical evolution.
Beyond the fundamental aspects of life’s origins, the study unveils significant implications for understanding disease processes linked to protein dynamics. Diseases such as cancer, neurodegenerative disorders, and even conditions like COVID-19 have been associated with aberrant phase separation events and protein misfolding. Understanding the Griffiths-like cellular phase could enhance our understanding of how proteins interact, compartmentalize, and occasionally malfunction, which could have profound implications for disease therapies.
Marcos Minicucci, a co-author of the study and a professor at UNESP’s Botucatu campus, emphasizes that the ability of proteins to form droplets mitigates or exacerbates disease states depending on the context. For instance, during tumorigenesis, certain proteins may be sequestered into droplets that alter their functionality, thereby influencing cell mutations.
This research exemplifies the importance of interdisciplinary collaboration between physics and biology in cracking complex biological problems. The combined expertise of physicists, biologists, and medical professionals has propelled this study forward, illustrating how bridging different scientific disciplines can yield deeper insights into fundamental questions.
Moreover, the relevance of the findings underscores an encouraging trend towards integrating physical principles into biological studies, which could lead to advancements in therapeutic strategies targeting phase separation. As researchers continue to explore these themes, the understanding of protein dynamics in cells will likely evolve, paving the way for innovative diagnostic and treatment approaches.
The exploration of liquid-liquid phase separation through the lens of condensed matter physics provides a novel framework for understanding complex biological processes. By proposing a Griffiths-like phase in cellular dynamics, Souza’s research not only enriches the physical understanding of protein organization but also poses essential questions regarding the origins of life and the pathology of diseases. As the lines between disciplines continue to blur, the potential for transformative discoveries in both medicine and evolutionary biology can only grow.

Leave a Reply